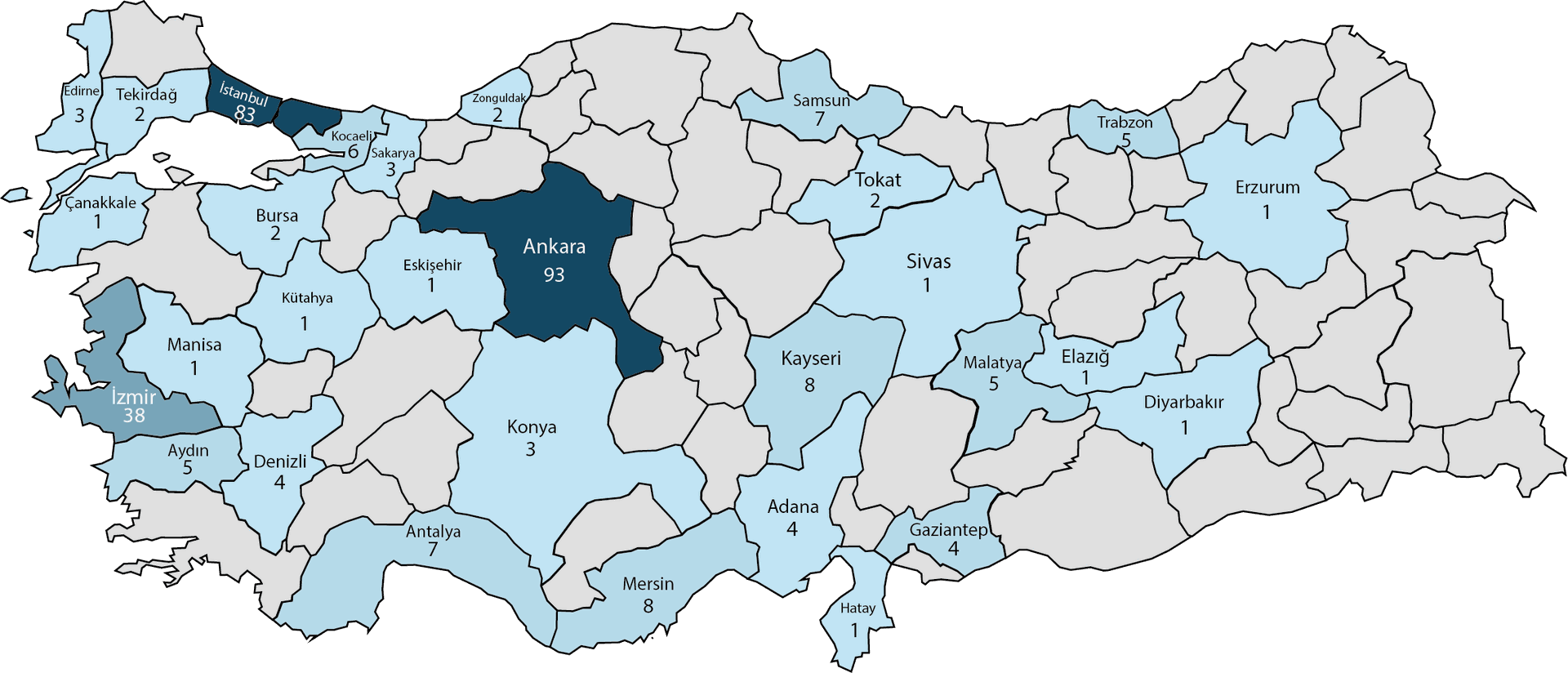
About us

Our Core Values
- Promoting Integrity in Quality
- Reliability
- Honesty
- Respect for People
- Continuous Improvement
- Responsibility to Society and Stakeholders
- Adherence to Business Ethics

Our Quality Policy
We are dedicated to providing superior site management services by adhering to stringent quality standards and regulatory requirements. Our quality policy emphasizes the identification, assessment, and mitigation of risks at every stage of the clinical research process. Accordingly, we commit to:
•Fulfilling, certifying, documenting, and continuously improving ISO 9001 requirements in all our activities that encompass service functions,
•Complying with national and international legal regulations and ethical standards,
• Enhancing collaboration to best meet the expectations of our stakeholders, and increasing our service diversity by closely following scientific and technological developments,
• Ensuring the best working environment for our employees, recognizing that customer satisfaction stems from employee happiness and satisfaction,
• Improving our employees' competencies and supporting continuous learning to enhance service quality,
• Implementing strong mentoring programs that pair bright minds with experienced mentors to provide guidance and support for acquiring the necessary knowledge and skills to succeed in clinical research,
• Working in mutual cooperation and trust with our suppliers and customers,
• Striving for excellence through regular review and improvement of our processes,
• Protecting the accuracy, reliability, and confidentiality of clinical research data
• Achieving financial growth by continuously increasing our market share,
• Ensuring our employees work in harmony in a safe and healthy environment without discrimination based on language, religion, race, gender, political opinion, sect, age, or physical condition.
We are committed to these principles.
Mehmet YILDIZ